c2h2 lewis dot structure|Lewis Structure for C2H2 (Ethyne) : Tagatay Wayne Breslyn. 771K subscribers. 197. 37K views 1 year ago. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Acetylene (Ethyne)). For the C2H2 structure use . Fractional odds are most common at British and Irish sportsbooks, as well as in horse racing. While this format doesn’t appear at U.S. sportsbooks, it can be helpful to understand if you want to .
c2h2 lewis dot structure,Wayne Breslyn. 771K subscribers. 197. 37K views 1 year ago. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Acetylene (Ethyne)). For the C2H2 structure use .c2h2 lewis dot structureHow to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne) It is helpful if you: Try to draw the C 2 H 2 Lewis structure before watching the video. Watch the video and .
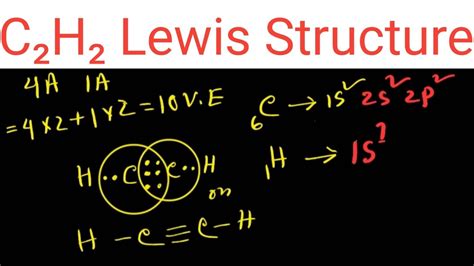
c2h2 lewis dot structure Lewis Structure for C2H2 (Ethyne) How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne) It is helpful if you: Try to draw the C 2 H 2 Lewis structure before watching the video. Watch the video and .You can see the lewis structure of C 2 H 2 in above figure and you can see it is a simple structure. Now, we are going to draw that C 2 H 4 lewis structure step by step. Steps of drawing the lewis structure of C 2 H 2. . chem101csub. 3.82K subscribers. Subscribed. 35. 16K views 10 years ago. If you are asking "help me with Lewis structures" then you came to the right place for free chemistry .
Learn how to draw the stable Lewis structure of acetylene (C2H2) with five steps and examples. Find out the valence electrons, lone pairs, formal charges, and octet rule of C2H2.

C2H2 Lewis Structure - How to draw the Electron Dot Structure for Ethene | Success in Chemistry. Chemical Bonding Main. Valence Electrons. Lewis Dot Structures. Formal .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. .
The Lewis structure of C2H2 helps us understand the geometry of the molecule. All the atoms here lie in the same plane, and there is no asymmetry in the molecule. As all the atoms are arranged .
The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two .2. H. 2. (Acetylene | Ethyne) Lewis Structure. C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the . A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. . Chemists normally .Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms.; Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Note that Hydrogen only needs two valence electrons . Step 3: Connect each atoms by putting an electron pair between them. Now in the C2H2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-hydrogen atoms. This indicates that these atoms are chemically bonded with each other in a C2H2 molecule. Step 4: Make the outer atoms stable.Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Suggest Corrections. 35. Q. Draw the electron dot structure of ethyne and also draw its. structural formula. Q. Question 30. Drawthe electron dot structure of ethyne and also draw its structural formula. Q. Draw electron dot structure of ethyne. Q. The total number of valence electrons in the acetylene or ethyne (C2H2) Lewis dot structure is 10. The molecular geometry or shape of C 2 H 2 is identical to its ideal electron pair geometry i.e., linear. The bonded atoms in C 2 H 2 form a mutual bond angle of 180°. The central C-atoms have sp hybridization in C 2 H 2.The C2H2 Lewis structure refers to the arrangement of atoms and electrons in a molecule of ethyne (C2H2) using Lewis dot diagrams. This involves representing each atom using its chemical symbol and drawing dots around it to represent its valence electrons.

This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Draw the Lewis structure for acetylene (C2H2). Be certain you include any lone pairs. Draw the Lewis structure for acetylene (C2H2). Be certain you include any lone pairs.
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.Lewis Structure for C2H2 (Ethyne) 5 Steps to Draw the Lewis Structure of C2H2 Step #1: Calculate the total number of valence electrons. Here, the given molecule is CH2. In order to draw the lewis structure of CH2, first of all you have to find the total number of valence electrons present in the CH2 molecule. (Valence electrons are the number of electrons present in the .PROBLEM 4.2.4 4.2. 4. Methanol, H 3 COH, is used as the fuel in some race cars. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO 2 and H 2 O when they burn. .
Hey Guys,In this video we are going to learn about the Lewis structure of C2H2. It is a chemical formula for Ethyne or Acetylene.To understand the Lewis stru.We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with .
Bonding in Ethane. In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3-hybridized, meaning that both have four bonds arranged with tetrahedral geometry.The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp 3 orbital from each of the carbons, .
Lewis Dot of Ethyne (Acetylene) C 2 H 2. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Acetylene is an unsaturated hydrocarbon with a triple bond. A step-by-step explanation of how to draw the C2H2Br2 Lewis Dot Structure (1,2-Dibromoethylene).For the C2H2Br2 structure use the periodic table to find the . Correct Correct Wrong. CCl 4 (A refrigerant) C 2 H 6 (Ethane) CHClCH (Bad layout, hydrogen and chlorine are central atoms) Note: Electronegativity values: C = 2.55; Cl = 3.16; H = 2.20. Step 2: Add up the valence electrons for each atom in the molecule. For example, H 2 O 2 H: 2 x 1 electron = 2 electrons. 1 O: 1 x 6 electrons = 6 electrons.
c2h2 lewis dot structure|Lewis Structure for C2H2 (Ethyne)
PH0 · Lewis Structure for C2H2 (Ethyne)
PH1 · How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
PH2 · C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle
PH3 · C2H2 Lewis structure, Molecular Geometry,
PH4 · C2H2 Lewis structure
PH5 · C2H2 Lewis Structure, Molecular Geometry
PH6 · C2H2 Lewis Structure Tutorial
PH7 · C2H2 Lewis Structure
PH8 · C2H2 Lewis Dot Structure + Geometry
PH9 · C2H2 (Ethyne) Lewis structure
PH10 · C2H2 (Acetylene